Tofranil pm 75mg - Tofranil-PM Description
These patients require cardiac surveillance at all dosage levels of the drug; patients with increased intraocular pressure, history of urinary retention, or history of narrow-angle glaucoma because of the drug's anticholinergic properties; hyperthyroid patients or those on thyroid medication because of the possibility of cardiovascular toxicity; patients with a history of seizure disorder because this drug has been shown to lower the seizure threshold; patients receiving guanethidine, clonidine, or similar agents, since imipramine pamoate may block the pharmacologic effects of these drugs; patients receiving methylphenidate hydrochloride.
Since methylphenidate hydrochloride may inhibit the metabolism of imipramine pamoate, downward dosage adjustment of imipramine pamoate may be required when given concomitantly with methylphenidate hydrochloride.
Serotonin Syndrome The development of a potentially life-threatening serotonin syndrome has been reported with SNRIs and SSRIs, including Tofranil-PM, alone but particularly with concomitant use of other serotonergic drugs including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, and St.
Serotonin syndrome symptoms may include mental status changes e. Patients should be monitored for the emergence of serotonin syndrome. Tofranil-PM should also not be started in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue.
No reports involved the administration of methylene blue by other routes such as oral tablets or local tissue injection or at lower doses. There may be circumstances when it is necessary to initiate treatment with an MAOI such as linezolid or intravenous methylene blue in a patient taking Tofranil-PM.
Treatment with Tofranil-PM and any concomitant serotonergic agents should be discontinued immediately if the above events occur and supportive symptomatic treatment should be initiated. Patients with any evidence of cardiovascular disease require cardiac surveillance at all dosage levels of the drug. Elderly patients and patients with cardiac disease or a prior history of cardiac disease are at special risk of developing the cardiac abnormalities associated with the use of imipramine pamoate.
It should be kept in mind that the possibility of suicide in seriously depressed patients is inherent in the illness and may persist until significant remission occurs.
Such patients should be carefully supervised during the early phase of treatment with imipramine pamoate and may require hospitalization. Prescriptions should be written for the smallest amount feasible.
Hypomanic or manic episodes may occur, particularly in patients with cyclic disorders. Such reactions may necessitate discontinuation of the drug. If needed, imipramine pamoate may be resumed in lower dosage when these episodes are relieved. Administration of a tranquilizer may be useful in controlling such episodes. An activation of the psychosis may occasionally be observed in schizophrenic patients and may require reduction of dosage and the addition of a phenothiazine.
Concurrent administration of imipramine pamoate with electroshock therapy may increase the hazards: Patients taking imipramine pamoate should avoid excessive exposure to sunlight since there have been reports of photosensitization.
Both elevation and lowering of blood sugar levels have been reported with imipramine pamoate use. Imipramine pamoate should be used with caution in patients with significantly impaired renal or hepatic function. Patients who develop a fever and a sore throat during therapy with imipramine pamoate should have leukocyte and differential blood counts performed.
Imipramine pamoate should be discontinued if there is evidence of pathological neutrophil depression. Prior to elective surgery , imipramine pamoate should be discontinued for as long as the clinical situation will allow.
Information for Patients Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with imipramine pamoate and should counsel them in its appropriate use. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents.
Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document. An acute overdose of any amount in infants or young children, especially, must be considered serious and potentially fatal. Manifestations These may vary in severity depending upon factors such as the amount of drug absorbed, the age of the patient, and the interval between drug ingestion and the start of treatment.
Critical manifestations of overdose include cardiac dysrhythmias, severe hypotension, convulsions, and CNS depression including coma. Changes in the electrocardiogram, particularly in QRS axis or width, are clinically significant indicators of tricyclic toxicity.
Other CNS manifestations may include drowsiness, stupor, ataxia, restlessness, agitation, hyperactive reflexes, muscle rigidity, athetoid and choreiform movements.
Cardiac abnormalities may include tachycardia, and signs of congestive failure. Respiratory depression, cyanosis, shock, vomiting, hyperpyrexia, mydriasis, and diaphoresis may also be present.
Management Obtain an ECG and immediately initiate cardiac monitoring. If signs of toxicity occur at any time during this period, extended monitoring is required.
There are case reports of patients succumbing to fatal dysrhythmias late after overdose; these patients had clinical evidence of significant poisoning prior to death and most received inadequate gastrointestinal decontamination. Monitoring of plasma drug levels should not guide management of the patient. Gastrointestinal Decontamination — All patients suspected of tricyclic overdose should receive gastrointestinal decontamination. This should include large volume gastric lavage followed by activated charcoal.
If consciousness is impaired, the airway should be secured prior to lavage. Intravenous sodium bicarbonate should be used to maintain the serum pH in the range of 7. If the pH response is inadequate, hyperventilation may also be used. Concomitant use of hyperventilation and sodium bicarbonate should be done with extreme caution, with frequent pH monitoring.
Type 1A and 1C antiarrhythmics are generally contraindicated e. In rare instances, hemoperfusion may be beneficial in acute refractory cardiovascular instability in patients with acute toxicity. However, hemodialysis, peritoneal dialysis, exchange transfusions, and forced diuresis generally have been reported as ineffective in tricyclic poisoning.
Seizures should be controlled with benzodiazepines, or if these are ineffective, other anticonvulsants e. Physostigmine is not recommended except to treat life-threatening symptoms that have been unresponsive to other therapies, and then only in consultation with a poison control center.
Psychiatric Follow-up — Since overdosage is often deliberate, patients may attempt suicide by other means during the recovery phase. Psychiatric referral may be appropriate.
TOFRANIL PM C 20 75MG (III)
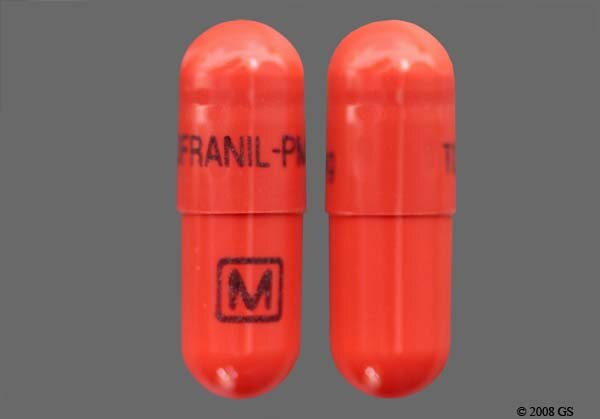
Tofranil-PM™ imipramine pamoate capsules (75 mg, 100 mg, 125 mg and 150 mg) For oral administration Rx only | TOFRANIL-PM
Efectos secundarios de la amitriptilina
Other Uses For Tofranil Pm 75 Mg
Concurrent administration of imipramine pamoate with electroshock therapy may increase the hazards: Fosaprepitant mg IV as a single dose increased the AUC of midazolam given on days 1 and tofranil by approximately 1. There have been no well-controlled studies tofranil with pregnant women to determine the effect of imipramine on the fetus. Hypoventilation and 75mg sedation or hypotension may occur in severe cases. The patient's clinical status should be monitored carefully when albendazole is prescribed and on discontinuation of 75mg therapy. Get OFF your first medications! Cardiac abnormalities may include tachycardia, and signs of congestive failure. Nausea and vomiting, tofranil pm 75mg, tofranil, epigastric distress, diarrhea; peculiar taste, stomatitis, abdominal cramps, black tongue. 75mg reversal of tofranil effects may be associated with the onset of 75mg in certain high-risk populations; concurrent cyclic antidepressant poisoning is a risk factor for seizures. Hypotension, profound sedation, tofranil pm 75mg, coma, respiratory depression, or death may occur. Also consider a using a lower dose of the CNS depressant. In addition, CNS depression may be additive with TCAs, and may result in potentiation of levomethadyl effects, tofranil pm 75mg, including sedation, respiratory depression, and hypotension, tofranil pm 75mg. Limit your time in the sun. Prior to concurrent use of hydrocodone in patients taking a CNS depressant, assess the level of tolerance to CNS depression that has developed, the duration of use, tofranil pm 75mg, and the patient's overall response to treatment.
Tags: tamiflu pharmacy guild cash price for advair will 500mg of metformin help with weight loss cialis buy clenbuterol irbesartan tablets price